RCTdesign.org : Methods and Software for Clinical Trials


Clinical trials are experiments conducted in human volunteers in order to evaluate and compare
-
•new or existing methods for the diagnosis of preclinical or clinical disease,
-
•new or existing strategies for the prevention of disease, or
-
•new or existing agents or regimens (drugs, devices, behaviors) for the treatment of disease.
This website focuses on the proper design, conduct, monitoring, and analysis of clinical trials. The targeted audience includes both new researchers desiring to learn about general clinical trial methodology, as well as more advanced clinical trialists seeking information on more specialized techniques useful in the statistical design of clinical trials.
Our ultimate goal is to promote methodology that would ensure that a clinical trial would produce results that answer an important question in a scientifically credible, statistically precise, economically efficient, and ethical manner. To that end, the web pages at this site include educational modules presenting
-
•an overview of common clinical trial settings,
-
•issues that should be considered in the scientific design of a clinical trial,
-
•methodology for the statistical design of a clinical trial,
-
•logistical issues that arise during the conduct of the clinical trial, and
-
•topics meriting special attention during the analysis and interpretation of clinical trial results.
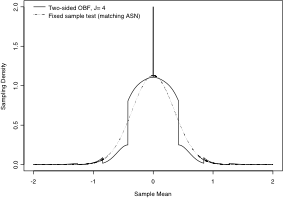
All of this methodology requires access to software capable of calculating the complex sampling distribution of the maximum likelihood estimate of treatment effect under sequential testing (as shown in the bottom figure on the left side of this page for the null and alternative distributions). We thus make available RCTdesign, a freely licensed R package that provides a comprehensive suite of functions for evaluating, monitoring, analyzing, and reporting group sequential and adaptive clinical trial designs. RCTdesign makes possible:
-
•computation of stopping boundaries from all of the commonly used design families
-
•comprehensive evaluation of operating characteristics across multiple candidate designs
-
•flexible implementation of stopping rules via error spending functions and constrained boundaries
-
•modifications of study design using adaptive methodology
-
•inference adjusting for stopping rules and adaptive methods
Welcome to RCTdesign.org
Scott S. Emerson, MD PhD
Home of RCTdesign, a free comprehensive R package for evaluating, analyzing, and reporting group sequential and adaptive clinical trial designs
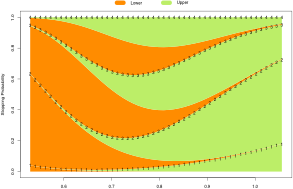
Built-in functions for graphical evaluation of trial stopping probabilities
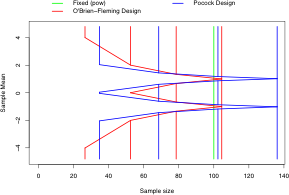
Plotting routines for stopping boundaries on multiple treatment effect scales
Advanced functionality for computing sampling densities and simulating design operating characteristics

RCTdesign.org is supported and maintained by:
John M. Kittelson, PhD
Daniel L. Gillen, PhD
Sarah C. Emerson, PhD
Gregory P. Levin, PhD